A Must-Read for Best Growth Subscribers!
Positive Developments in 2023:
Phase 2b Data for BNC210 in PTSD: Bionomics announced positive results from the Phase 2b ATTUNE trial in PTSD, showing a reduction in total symptom severity and improvements in multiple symptom clusters. A discussion with the FDA about these results is anticipated in Q2 2024.
End of Phase 2b Meeting for BNC210 in SAD: Following the Phase 2b PREVAIL study, Bionomics had a successful meeting with the FDA, setting the stage for a registrational study in Social Anxiety Disorder (SAD).
Strategic Partnerships: The company is engaged in discussions for potential strategic partnerships for multiple pipeline assets and is exploring options to advance clinical development plans for BNC210 in both PTSD and SAD.
Key Highlights and Milestones:
BNC210’s Role in CNS Disorders: BNC210, a first-in-class α7 nicotinic receptor modulator, showed promise in treating PTSD and SAD. It could become the first “as-needed” non-sedating, non-habit-forming anxiolytic for SAD.
Alzheimer’s and Cognitive Impairment: In partnership with MSD, Bionomics is developing α7 PAMs for cognitive disorders like Alzheimer’s disease, with two clinical candidates in late Phase 1 development.
Corporate Developments: Bionomics expanded its U.S.-based team, delisted from the Australian Stock Exchange, efficiently allocated capital, and continued strengthening its intellectual property portfolio.
Financial Position: The company has no outstanding debt, convertible securities, or warrants and maintains support from institutional investors.
2024 Outlook and Anticipated Milestones:
Potential Catalysts
Phase 3 Study in SAD: Bionomics plans to initiate the first Phase 3 study of BNC210 in patients with SAD.
Discussions with FDA on PTSD Study: The company anticipates discussing a late-stage study of BNC210 in PTSD with the FDA, with an update expected by the end of Q2 2024.
Breakthrough Therapy Designation: Bionomics is exploring a request for Breakthrough Therapy designation in PTSD in Q2 2024.
Strategic Partnerships: The company aims to establish strategic partnerships for BNC210.
Conclusions from this corporate update:
Bionomics achieved significant milestones in 2023, particularly with the positive results of BNC210 in PTSD and SAD. Looking ahead to 2024, the company is poised for further growth with strategic plans, including initiating Phase 3 studies, pursuing regulatory discussions, and exploring partnerships. Developing innovative treatments for CNS disorders with high unmet medical needs (Large Markets!)
Source article: Original and full company update
Bionomics (BNOX) Strategic partnership with Merck & Co, Inc
The partnership with Merck & Co. on α7 PAMs, with a potential value of $500 million in milestones plus royalties, exemplifies the high-stakes nature of CNS drug development and the significant revenue potential for successful therapies.
Share Statistics
Current Price a/o the close 01/24/2024: 0.99/Share
52-Week Performance:
52 Week High: $7.71
52 Week Low: $0.93
Moving Averages:
50-Day Moving Average: $1.41
200-Day Moving Average: $2.09
Trading Volume:
Average Volume (3 months): 230.33k shares
Average Volume (10 days): 288.59k shares
Share Statistics:
Shares Outstanding: 10.26 million
Float: 8.81 million shares
Percentage Held by Institutions: 32.25%
Sources: Yahoo finance and dilution tracker
Summary
Bionomics (NASDAQ: BNOX) – FDA Announcements and Additional Potential Catalysts with Timeframes
Background:
Bionomics Limited, a clinical-stage biotechnology company, has made significant progress with its lead asset BNC210, aimed at treating Social Anxiety Disorder (SAD). Following a successful End-of-Phase 2 (EoP2) meeting with the U.S. Food and Drug Administration (FDA) in September 2023, the company is poised to advance BNC210 into Phase 3 registrational studies.
Key FDA Meeting Outcomes:
Agreement on Phase 3 Clinical Program: Bionomics and the FDA have agreed on conducting two randomized, placebo-controlled studies with a single administration of BNC210 during a public speaking task.
Primary Efficacy Endpoint: The Subjective Units of Distress Scale (SUDS) measured during a public speaking challenge will be the primary efficacy endpoint.
Doses for Phase 3 Studies: Specific doses of BNC210 to be studied in Phase 3 have been agreed upon.
Sample Size and Design Elements: The company has aligned with the FDA on the sample size assumptions based on the PREVAIL findings and the design of the open-label safety study required for the New Drug Application (NDA).
Safety Database Size: An agreement on the size of the safety database to support the NDA has been reached.
Nonclinical Toxicology Studies: Requirements for nonclinical toxicology studies needed for the NDA have been established.
Anticipated Timeframes and Potential Catalysts
Initiation of Phase 3 Program: Bionomics plans to begin the Phase 3 program in Q1 2024. This marks a significant milestone and potential catalyst for the company as it moves closer to potential commercialization.
Progress of Phase 3 Studies: The commencement and progress of the Phase 3 studies throughout 2024 will be crucial for Bionomics. Positive interim results or updates could serve as catalysts for investor interest and stock movement.
Strategic Partnerships: Potential partnerships or collaborations related to BNC210, especially in the wake of positive Phase 2 results, could emerge as additional catalysts.
Regulatory Milestones: Any subsequent positive interactions or feedback from the FDA, including potential breakthrough therapy designations or priority reviews, would be significant for the company’s trajectory.
Expansion in Other Indications: Given BNC210’s potential in multiple anxiety disorders, developments in expanding its application to other related CNS disorders could provide further upside.
Conclusion
Bionomics is positioned for a pivotal year in 2024, with the advancement of BNC210 into Phase 3 studies for SAD being a key focus. The alignment with FDA on critical aspects of the Phase 3 program underscores the company’s commitment to addressing the unmet needs in the treatment of SAD. The initiation of these studies, along with potential regulatory and strategic milestones, are poised to serve as significant catalysts for Bionomics in the near future.
Daily Chart
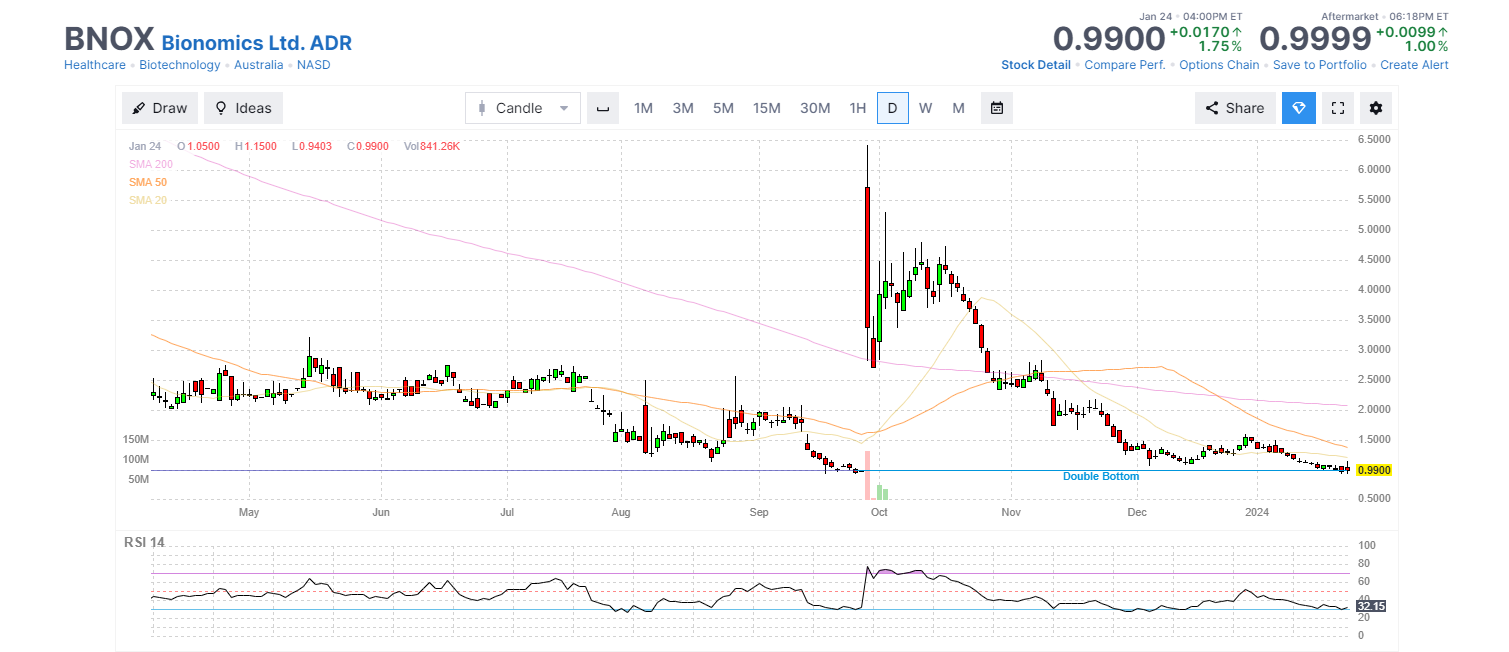
Chart source: Finviz
Stay tuned throughout 2024 for future updates on BNOX.
Your friend,
Steve Macalbry
Senior Editor, BestGrowthStocks.Com
Disclosures and About Us
Best Growth utilizes a revolutionary fusion of AI-powered analytics and human-led research to distill the vast and complex world of stock trading down to its purest essence. Our proprietary AI, meticulously designed to perform both fundamental and sentiment analysis, scrutinizes an extensive array of data points and trends, providing deep, insightful, and timely market perspectives. This technology operates in concert with our seasoned research team, who apply years of industry knowledge and expertise to refine our AI’s results. The culmination of this unique blend of cutting-edge technology and human expertise is the weekly issuance of one to two stock ideas, carefully selected to offer what we believe will yield the most substantial near-term results (a few days to a few months), thus paving the way for intelligent trading that delivers tangible value. Available exclusively by email or SMS/text message via our newsletter.
Best Growth is your ultimate destination for reliable and insightful stock news and analysis. We are dedicated to providing you with the latest updates and in-depth coverage of the world’s most promising growth stocks. Our experienced financial experts and market analysts work tirelessly to bring you the most accurate and up-to-date information.
At Best Growth, we understand that the investing world can be complex and intimidating. That’s why our primary goal is to simplify the process for you, offering easy-to-understand news articles, market reports, and expert commentary. Whether you are a seasoned investor or just starting your journey in the stock market, we strive to cater to your needs by presenting information in a clear and concise manner.
Our commitment to excellence extends to the quality of our content. We pride ourselves on delivering accurate, objective, and fact-based news and analysis. Our team follows a rigorous editorial process to ensure the highest standards of journalistic integrity.
At Best Growth, we believe in the power of knowledge and education. We go beyond simply reporting stock news; we provide third-party educational resources and investment guides to help you navigate the complexities of the market. We aim to empower you with the knowledge and tools necessary to achieve your financial goals.
We also understand that time is of the essence when it comes to stock analysis and news. That’s why we offer a user-friendly platform that ensures you have quick and easy access to the latest information. be sure you are subscribed to our newsletter and text alerts. Whether you prefer browsing our website or accessing our content through our free or premium subscription, we strive to make your experience seamless and convenient.
We value our community of readers and investors, and we encourage active engagement and participation. Feel free to share your thoughts and contribute to the discussions on our platform (coming soon). We believe that a vibrant and diverse community of investors can enhance the learning experience for everyone involved.
Thank you for choosing Best Growth as your go-to source for reliable stock news and analysis. We are committed to serving you with excellence and helping you stay ahead in the dynamic world of investing. Join us on this exciting journey toward financial growth and prosperity!
Sponsored Content Disclosure – In accordance with the Securities Act Section17 (b)
Transparency is very important to us. Please read this disclaimer in its entirety to fully understand this segment of our business model.
BestGrowthStocks.com is a wholly owned subsidiary of Media Source LLC, herein referred to as MS LLC.
This website / media webpage is owned, operated, and edited by Media Source LLC. Any wording found on this website / media webpage or disclaimer referencing to “I” or “we” or “our” or “MS LLC” refers to Media Source LLC. This website / media webpage is a paid advertisement, not a recommendation nor an offer to buy or sell securities. This portion of our business model is to be financially compensated to market and promote public companies. By reading our website / media webpage you agree to the terms of our disclaimer, which are subject to change at any time.
Our sponsored reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use. Part of MS LLC’s business model is to receive financial compensation to promote public companies in an investor relations capacity. To conduct investor relations advertising, marketing and publicly disseminate information not limited to our Websites, Email, SMS, Push Notifications, Influencers, Social Media Postings, Ticker Tags, Press Releases, Online or Phone Interviews, Podcasts, Videos, Audio Ads, Banner Ads, Native Ads, Responsive Ads. This compensation is a major conflict of interest in our ability to be unbiased regarding the publicly traded entities mentioned. Therefore, this communication should be viewed as a commercial advertisement only. Note, we periodically conduct interviews and issue stock alerts that we are not compensated for, these are purely for the purpose of building our brands and other portions of our business model. We have not investigated the background of the hiring third party or parties. The third party, profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to hurt share prices. Frequently companies profiled in our alerts may experience a large increase in volume and share price during the course of investor relations marketing, which may end as soon as the investor relations marketing ceases. Our emails may contain forward-looking statements, which are not guaranteed to materialize due to a variety of factors. We do not guarantee the timeliness, accuracy, or completeness of the information on our website / media webpage. The information in our website / media webpage is believed to be accurate and correct but has not been independently verified and is not guaranteed to be correct.
Please Note: MS LLC and its employees are not a registered investment advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold MS LLC, its operator’s, owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information in our website / media webpage is believed to be accurate and correct but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct. Furthermore, MS LLC often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is certainly possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications. The information in our disclaimers is subject to change at any time without notice. Some of our claims regarding gains could be based on intra-day, pre-market and after-hours trading data.
All information on featured companies is provided by the companies profiled or is available from public sources and MS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead, MS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
MS LLC is compliant with the Can Spam Act of 2003. MS LLC does not offer such advice or analysis, and MS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in small and micro-cap growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investors investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a safe harbor in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be forward looking statements. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quote; may, could, or might occur. Understand there is no guarantee past performance will be indicative of future results.
In preparing this publication, MS LLC has relied upon information supplied by its customers, publicly available information, and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The owners and operators of this website have been compensated up to seventeen thousand five hundred dollars cash via bank wire by Amplifyir inc. for a 1-2 day investor awareness campaign of BNOX to begin on 01-25-2024 and end on 01-25 or 1-26-2024. We do not hold any form of equity in BNOX. The advertisements in this website are believed to be reliable, however, MS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement.
MS LLC is not responsible for any claims made by the companies advertised herein, nor is MS LLC responsible for any other promotional firm, its program or its structure.
