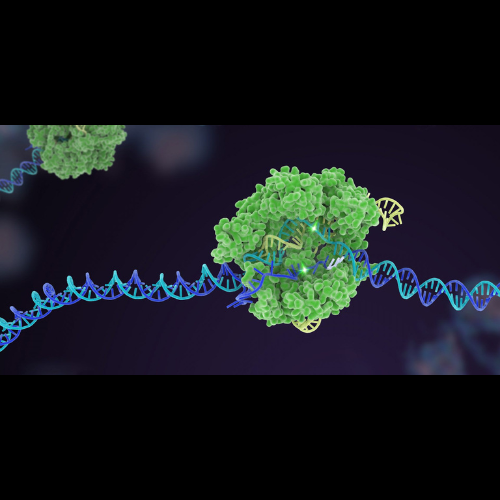
The U.S. Food and Drug Administration has recently approved the first gene-editing treatment, Casgevy, for sickle cell disease. This one-time treatment, developed by Vertex Pharmaceuticals (NASDAQ: VERX) and CRISPR Therapeutics (NASDAQ: CRSP), utilizes the Nobel Prize-winning CRISPR technology to treat sickle cell disease, a blood disorder affecting about 100,000 Americans. Casgevy is a cell-based gene therapy approved for patients 12 years of age and older with recurrent vaso-occlusive crises. The treatment involves modifying patients’ hematopoietic (blood) stem cells using CRISPR/Cas9 technology. Casgevy comes with a price tag of $2.2 million per patient, and it is expected to be available in early 2024.
The approval of Casgevy marks a significant milestone in the field of gene therapy and offers hope to thousands of individuals affected by sickle cell disease. The treatment’s use of CRISPR technology, which allows for precise gene editing, represents a groundbreaking approach to addressing genetic disorders. This development has the potential to transform the treatment landscape for sickle cell disease and may pave the way for further advancements in gene therapy for other genetic conditions.
The approval of Casgevy also highlights the rapid progress of gene-editing technologies, from their initial discovery to becoming approved treatments. The use of CRISPR to edit the DNA of a patient’s stem cells and eliminate the gene responsible for sickle cell disease represents a significant advancement in the quest for effective and innovative treatments for genetic disorders.
The high cost of Casgevy, priced at $2.2 million per patient, has sparked discussions about the accessibility and affordability of such advanced treatments. While the approval of Casgevy is a momentous achievement, questions remain about the long-term effectiveness and potential off-target effects of the CRISPR gene-editing approach. Ongoing research and monitoring will be crucial to further evaluate the safety and efficacy of Casgevy and similar gene-editing treatments.
by Steve Macalbry
Senior Editor,
BestGrowthStocks.Com
Disclaimer: This article is intended for informational purposes only. It should not be considered financial or investment advice. The author does not hold any form of equity in the securities mentioned in this article. Always consult with a certified financial professional before making any financial decisions.